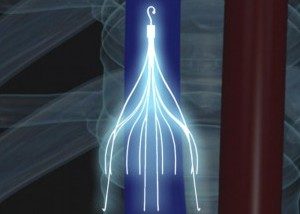
IVC Filters May Increase Mortality Rate in Patients Over 65
When an elderly individual suffers from a pulmonary embolism (PE), approximately one in six of them receive inferior vena cava (IVC) filters to decrease their risk of having subsequent PE. PE occurs when an artery in the lungs becomes blocked by a blood clot, which…